
The Cannabinoid Factories of the Future
Cannabis plants produce hundreds of interesting compounds, but could microorganisms provide a more efficient means for their mass production?
Synthia (formally known as Mycoplasma laboratorium) was the prodigal child of synthetic biology – a living cell comprising the outer membrane of a hollowed-out Mycoplasma capricolum and a Mycoplasma mycoides genome synthesized completely from digitized sequence information (1). This science may sound more aligned with the plot of a Philip K. Dick novel than reality, but synthetic biology was pushing these boundaries as early as 2010.
Since then, researchers have extended the frontiers of this novel field. E. coli cells have been engineered with the ability to produce synthetic proteins from a genetic code containing manmade nucleotide bases – essentially re-sketching the blueprints of life – and we have also equipped living cells with computational capabilities, such as logic, memory and problem-solving. Though impressive, these breakthroughs tell us very little about what synthetic biology is – or what it has to offer in practical terms.
Simply put, synthetic biology is an interdisciplinary field that applies engineering principles to the construction of biological systems that fulfil prespecified functions (for example, the synthesis of fuels or vaccines) based on carefully designed genetic circuits. The gene editing approaches central to this field are adaptable, and are being harnessed to engineer microorganisms able to rival classical ways of providing commodities in many spheres.
In the medical cannabis industry, for example, microbes have been engineered with the capacity to produce a number of cannabis compounds – largely cannabinoids like CBD and cannabigerol (CBG), but also a number of further substances. Yet, these compounds represent only a fraction of the potentially therapeutic compounds found in the plant. In this sense (and others yet to be discussed), we have merely scratched the surface of synthetic biology’s potential in this space.
The medical cannabis community has taken small steps towards a biosynthetic future – but what giant leap is needed before we usher in the age of cannabis’ synthetic overlords? And why would we bother in the first place?
The biosynthesizers

Jay Keasling
A household name in the field of synthetic biology, Jay Keasling has spent 27 years at University of California, Berkeley, engineering microbes. The success stories in his portfolio? Biosynthesizing taxol (an anti-cancer drug) and artemisinin (an anti-malarial drug precursor), and an extensive number of biofuels. Keasling has also been involved in numerous business ventures, co-founding both Amyris and Lygos. The recipient of numerous awards in both bioengineering and innovation, Keasling says he is looking forward to focusing on increasingly elusive molecules as he embarks on future ventures including the biosynthesis of key cannabis compounds.

Anna Shlimak
Head of Investor Relations and Communications at Cronos Group – a global cannabinoid company committed to building disruptive intellectual property by advancing cannabis research and product development – Anna Shlimak plays a crucial role in communicating the company’s cannabinoid biosynthesis programs. Working with Ginkgo Bioworks, they are making waves in the field of cannabinoid biosynthesis by using the expertise of both organizations to tackle key issues, such as scalability, access to rare cannabinoids and economic sustainability.

Jason Poulos
Librede is a company with a clear goal: to harness the therapeutic potential of nature. Cannabinoids represent valuable potential in this endeavor, providing a window of opportunity that CEO Jason Poulos was not prepared to miss. After obtaining his PhD in bioengineering from the University of California, Poulos wasted no time in immersing himself in the upper echelons of biotech business, soon developing the world’s first yeast-based cannabinoid production platform alongside Anthony Farina. Today, his focus is on establishing a wide network of collaborators with whom to develop new methods for synthesizing molecules.
Biosynthetic superiority?
Thinking on the latter question of why, Anna Shlimak, Head of Investor Relations and Communications for Cronos Group, provided a clear rationale. “The potential uses of cannabinoids are vast, but the key to successfully bringing cannabinoid-based products to market lies in creating reliable, consistent, and scalable production capability across the full spectrum of cannabinoids.”
There are three basic ways to go about producing any compound at scale: synthetic chemistry, classical agriculture, or cellular agriculture (an umbrella term under which synthetic biology sits). When it comes to cannabis compounds, classical agriculture is clearly the historical method of choice, but the approach suffers from clear challenges. In fact, agricultural cultivation is burdened by a number of shortcomings.
“The agricultural approach is not economically effective or environmentally sustainable,” says Jason Poulos, CEO of Librede Inc. “Hundred-thousand-foot greenhouses are needed to meet the demand for cannabinoids, which require not only colossal amounts of electricity to power, but also huge amounts of fertilizers and pesticides to produce a quality product.” The negative effects of these compounds are widely documented, and span from depleting key insect populations to damaging human health and the environment.
“Producing these compounds in yeast, or another applicable microorganism, has the potential to reduce costs, stabilize the supply chain against issues such as weather variability, and increase accessibility,” says Poulos. “In the case of molecules like CBD, an anti-epileptic medicine, accessibility should be our primary concern.”

Crop growth is affected by many factors, and even slight perturbations can lead to changes in the yield of a target compound by 10 percent or more. In the interest of stabilizing supply chains and increasing accessibility, as Poulos suggests, we must be able to anticipate the amount of a compound obtainable from a given production process.
Accessibility is a key theme in biosynthetic discussions – but expanding the portfolio of medical compounds available through cannabis represents another important goal. Biosynthesis allows us to capitalize on promiscuous metabolic pathways (those able to process one of a number of substrates into different products) to produce unnatural analogues of target compounds; the cannabinoid pathway can produce many such analogues when fed variants of hexanoic acid.
Talking of these possibilities, Jay Keasling of the University of California, Berkeley, suggests that synthetic biology carries the major advantages of both synthetic chemical and biochemical manufacturing approaches in a single package. “The great thing about synthetic biology is that it provides a relatively simple route for producing natural molecules with stereochemical centers, and also facilitates the synthesis of molecular variants,” he says. “This covers some of the weak points of synthetic chemistry and biochemical methods, respectively.”
Rise of the biosynthesizers
With such advantages in mind, it should come as no surprise that big names across industry and academia are hoping to harness this untapped potential – our contributors included. For Shlimak, this is evidenced by Cronos Group’s recent collaboration with Ginkgo Bioworks (a customized microbe design company); for Poulos, we need only look at Librede’s history of biosynthetic cannabinoid patents; and for Keasling, recent success in synthesizing cannabinoids and their unnatural analogues in yeast speaks for itself (2).
Accordingly, Poulos highlights extreme levels of competition. “Every day there seems to be a new company wanting to enter this space. We’re even seeing publicly traded companies take interest, but these groups quickly realize that saying is easier than doing,” he says. Librede is developing a hefty patent portfolio to beat off competitors; the most recent of these covered an approach for producing THC acid (THCA) in yeast, with existing patents in place for methods concerned with the biosynthesis of CBD and CBG. The existence of so much competition so soon after changes to legislation points to rapid and continued progress for cannabinoid biosynthesis in the industrial sphere.
Feats elsewhere in academia also speak to a rapidly advancing subfield. A team from TU Dortmund University (Germany) have engineered yeast with the ability to conduct whole-cell CBGA to THCA bioconversion (3), and, in August, Kevin Rea and colleagues from the University of Guelph (Canada) reported the successful biosynthesis of anti-inflammatories cannflavin A and B, also in yeast (4).
Keasling attributes these advances to a number of breakthroughs in molecular science, including DNA synthesis. Labs today are able to bypass cloning genes out of the cannabis plant completely, and can also alter the codons within to modify genetic expression as required. What’s more, automation and robotics streamline the practical work itself, and dramatic advances in analytical technologies like mass spectrometry mean that we can analyze the biosynthesized products with high throughput.
With the aim of capitalizing on such advances to biosynthesize eight target cannabinoids, Cronos Group have recently purchased a state-of-the-art facility, which will operate as “Cronos Fermentation.” Shlimak was happy to share a few details. “The facility includes fully equipped laboratories covering microbiology, organic and analytical chemistry, quality control and method development, as well as two large microbial fermentation areas with a combined production capacity of 102,000 liters. Plus, three downstream processing plants, and bulk product and packaging capabilities,” she says.
Such facilities should provide the fermentation and manufacturing capabilities needed for Cronos Group to take full advantage of the work currently underway with Ginkgo. “Simply put, the process will be similar to that of yeast-based beer fermentation,” says Shlimak, but current gaps in our knowledge of cannabinoid biosynthesis and the relevant technologies mean we have a way to go before upscaling to volumes as striking as 102,000 liters.
Blockers to biosynthetic success
To upscale production, those in pursuit of cannabis compound biosynthesis must focus their expertise and ingenuity on three significant objectives: improving technology, reducing costs, and championing success stories.
Keasling believes that translating a biosynthetic cannabinoid product from laboratories to the market will be a crucial first step. “Getting a product into the market and placing it into the hands of consumers is essential because it will act as proof of concept to the public and evidence that this area of research was worth the investment,” he says. Such success stories would likely then spur on further research; as Shlimak says, “Providing consistent and reliable products will facilitate additional innovation in this space by bringing new formats and technologies to the market.”
A change in public perception would also help. The term genetically modified organism seems to incite confusion and fear even today, and an improved public understanding of its meaning would ease the movement of biosynthesized cannabinoids into the market.
In terms of improving technology, Poulos believes the field would benefit most from advances that focus on screening for the cannabinoids being biosynthesized, rather than on the genetic means for their production: “We can easily incorporate enzymes from countless organisms into a microbe of interest by simply printing the DNA encoding them. The problem is: how do we quickly test that the resulting cannabinoids are really there? It’s not as simple as observing a change in color.”
Poulos argues that such screening tools could be useful not just for cannabinoid biosynthesis, but also in biosynthetic efforts to produce classical pharmaceutical products, biofuels, and so on. “If I could snap my fingers tomorrow and have any breakthrough in this field in front of me, improved screening would be it,” he says.
In addition, advances in the development of stable cell lines suitable for use on an industrial scale and downstream processing for cannabis compound extraction and purification are also needed. Low-cost materials will be central to these endeavors, which underscores perhaps the most important requirement for the field moving forward – reduced costs.
Though the chemical synthesis of cannabis compounds is incredibly expensive, costing around $40,000–70,000 per kilogram, Keasling suggests that costs as low as $100 per kilogram could be accomplished through microbial synthesis. With a wholesale price for CBD of around $5,000 per kilogram, reducing the price of biosynthesis represents a route to maximize yield and profit for the companies working in this space. Perhaps the most lucrative line of attack, however, will be the biosynthesis of lesser-known cannabis compounds with medical applications, which currently hold wholesale values of up to around $60,000 per kilogram.
Biosynthetic blockbusters of tomorrow
Cannabis compounds other than those typically mentioned in medical discussions are an important target for biosynthesis. Keasling refers to these as potential “blockbuster” compounds, and is excited to explore the utility of yeast to synthesize them. “I’m really interested in rare cannabinoids, but we don’t know much about them because of the small quantities they’re produced in. Yeast provides a great platform to further study these compounds, and I’m really excited to see these experiments unfold,” he says.
These compounds also represent an important target for Cronos Group. “Our platform with Ginkgo will hopefully grant us access to cannabinoids present at low quantities in the plant, meaning that they are economically impractical, difficult or impossible to extract from agricultural sources. These could be medically important, and potentially very valuable,” says Shlimak.
For Poulos, future ventures will focus on so-called “stem cell cannabinoids” CBGA and cannabigerovarinic acid – major cannabinoid precursors (see Figure 1 for an overview of their metabolism). Librede is already producing these compounds in quantities of hundreds of grams and is focusing on process improvements to lower costs. Next on their hit list: further compounds such as THC, CBD, cannabichromene, and tetrahydrocannabivarin.
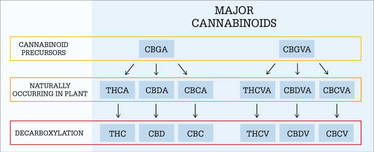
Cannabinoid metabolism from major precursors (adapted from a diagram provided by Cronos Group)
Despite the potential and value of lesser-known and little-understood cannabinoids, the current drive of the field is clear. “From a pharmaceutical standpoint, the primary molecule of interest is CBD right now,” says Poulos.
Beyond pharma, we should also expect to see applications for biosynthesized cannabinoids in nutraceuticals, beer, cosmetics, and any other markets that deem them useful. The demand for this research is clear – now it’s up to researchers to continue pushing the boundaries.
Will we see cannabis greenhouses replaced completely by fermentation tanks? Maybe not anytime soon. But the case for cannabinoid biosynthesis – both in mass production and drug discovery – is certainly compelling.
- DG Gibson et al., “Creation of a bacterial cell controlled by a chemically synthesized genome”, Science, 329, 52 (2010). DOI: 10.1126/science.1190719
- X Luo et al., “Complete biosynthesis of cannabinoids and their unnatural analogues in yeast”, Nature, 576, 123 (2019). DOI: 10.1038/s41586-019-0978-9
- B Zirpel et al., “Optimization of Δ9-tetrahydrocannabinolic acid synthase production in Komagataella phaffii via post-translational bottleneck identification”, J Biotechnol, 272-273, 40 (2018). DOI: 10.1016/j.jbiotec.2018.03.00
- KA Rea et al., “Biosynthesis of cannflavins A and B from Cannabis sativa L”, Phytochemistry, 164, 12 (2019). DOI: 10.1016/j.phytochem.2019.05.009
- X Luo et al., “Complete biosynthesis of cannabinoids and their unnatural analogues in yeast”, Nature, 576, 123-126 (2019). DOI: 10.1038/s41586-019-0978-9
I've always wanted a career in which I could practice my creativity, even when I worked on the assembly line in a fish factory. At one time, I channeled this need into dance, drawing, poetry and fiction, and I still do most of these things. But, following completion of my MSc(Res) in Translational Oncology and time working in labs and as a Medical Writer for major pharmaceutical companies, I'm happy to find myself in a career that allows me to combine my creative side with my scientific mind as the Deputy Editor of The Analytical Scientist.












